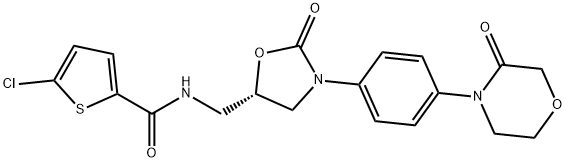
Rivaroxaban
|
- ₹0
- Product name: Rivaroxaban
- CAS: 366789-02-8
- MF: C19H18ClN3O5S
- MW: 435.88
- EINECS:685-132-2
- MDL Number:MFCD11974010
- Synonyms:(S)-Rivaroxaban;5-Chloro-N-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl]-2-thiophenecarboxamide;BAY 59-7939;Rivaroxaban Isomer;Rivaroxaban;5-Chloro-N-(((5S)-2-oxo-3-(4-(3-oxomorpholin-4-yl)phenyl)-1,3-oxazolidin-5-yl)methyl)thiophene-2-carboxamide;2-Thiophenecarboxamide, 5-chloro-N-[[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-5-oxazolidinyl]methyl];(S)-5-Chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)-oxazolidin-5-yl)methyl)thiophene-2-carboxami
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :228-229°C
Boiling point :732.6±60.0 °C(Predicted)
Density :1.460±0.06 g/cm3(Predicted)
storage temp. :Inert atmosphere,2-8°C
solubility :insoluble in H2O; insoluble in EtOH; ≥13.9 mg/mL in DMSO with gentle warming
form :solid
pka :13.36±0.46(Predicted)
color :White to off-white
InChI :InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
InChIKey :KGFYHTZWPPHNLQ-AWEZNQCLSA-N
SMILES :C1(C(NC[C@@H]2OC(=O)N(C3=CC=C(N4CCOCC4=O)C=C3)C2)=O)SC(Cl)=CC=1
Boiling point :732.6±60.0 °C(Predicted)
Density :1.460±0.06 g/cm3(Predicted)
storage temp. :Inert atmosphere,2-8°C
solubility :insoluble in H2O; insoluble in EtOH; ≥13.9 mg/mL in DMSO with gentle warming
form :solid
pka :13.36±0.46(Predicted)
color :White to off-white
InChI :InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
InChIKey :KGFYHTZWPPHNLQ-AWEZNQCLSA-N
SMILES :C1(C(NC[C@@H]2OC(=O)N(C3=CC=C(N4CCOCC4=O)C=C3)C2)=O)SC(Cl)=CC=1
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | |||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
Rivaroxaban is an orally active, direct inhibitor of Factor Xa (Ki = 0.4 nM), which is a crucial component of the intrinsic and extrinsic pathways of the blood coagulation cascade. It demonstrates >10,000-fold greater selectivity for Factor Xa compared to other related serine proteases. In various animal arterial and venous thrombosis models, rivaroxaban is reported to inhibit thrombin formation without prolonging bleeding time and has been approved for clinical use as an anticoagulant in the prevention of stroke and the treatment of venous thromboembolisms.More related product prices
Dabigatran Dabigatran etexilate Apixaban MK-2206 2HCl 1268524-70-4 Selumetinib Bortezomib 351-35-9 5-CHLOROTHIOPHENE-2-CARBOXYLIC ACID 3-MORPHOLINONE, 4-[4-[(5S)-5-(AMINOMETHYL)-2-OXO-3-OXAZOLIDINYL]PHENYL]- 141942-85-0 42518-98-9 (S)-4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one.HCl 161596-47-0 4-(4-AMINOPHENYL)MORPHOLIN-3-ONERelated product price
- 1268524-70-4
₹21390.2-75894.08 - Bortezomib
₹12940 - 351-35-9
₹3900-10932






