(R)-(+)-2-Methyl-2-propanesulfinamide
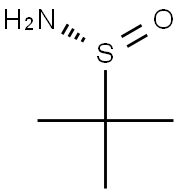
- CAS No.
- 196929-78-9
- Chemical Name:
- (R)-(+)-2-Methyl-2-propanesulfinamide
- Synonyms
- (R)-2-METHYLPROPANE-2-SULFINAMIDE;(R)-TERT-BUTANESULFINAMIDE;(R)-2-METHYL-2-PROPANESULFINAMIDE;Trityl glycidyl ether;(R)-N-tert-Butanesulfinamide;DASO-002;Borax LR;R-(+)-TBSA;-2-propanesuL;Nicotinamide Impurity 18
- CBNumber:
- CB2765464
- Molecular Formula:
- C4H11NOS
- Molecular Weight:
- 121.2
- MOL File:
- 196929-78-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 9:43:24
| Melting point | 103-107 °C(lit.) |
|---|---|
| Boiling point | 220.0±23.0 °C(Predicted) |
| alpha | 4 º (c=1, CHCl3 + amylenes) |
| Density | 0.903 g/mL at 25 °C |
| refractive index | 4 ° (C=1, CHCl3) |
| Flash point | -17℃ |
| storage temp. | 2-8°C |
| solubility | Soluble in chloroform, methanol, tetrahydrofuran, dichloromethane, dimethyl sulfoxide and most organic solvents. |
| form | Powder |
| pka | 10.11±0.50(Predicted) |
| color | White, light pink, light yellow to brown |
| optical activity | [α]22/D +1.0°, c = 0.5% in chloroform |
| Stability | store cold |
| InChIKey | CESUXLKAADQNTB-SSDOTTSWSA-N |
| CAS DataBase Reference | 196929-78-9(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302-H319-H335-H351 | |||||||||
| Precautionary statements | P210-P280-P301+P312+P330-P305+P351+P338-P370+P378-P403+P235 | |||||||||
| Hazard Codes | Xi,Xn,F | |||||||||
| Risk Statements | 11-19-36/37-40-36/37/38 | |||||||||
| Safety Statements | 22-24/25-36/37-26-16-36/37/39 | |||||||||
| RIDADR | UN 2056 3 / PGII | |||||||||
| WGK Germany | 3 | |||||||||
| Hazard Note | Irritant/Keep Cold | |||||||||
| TSCA | No | |||||||||
| HS Code | 29309090 | |||||||||
| NFPA 704 |
|
(R)-(+)-2-Methyl-2-propanesulfinamide price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 737941 | (R)-(+)-2-Methyl-2-propanesulfinamide solution 1?M in THF, contains BHT as inhibitor | 196929-78-9 | 10ML | ₹1569.4 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 737941 | (R)-(+)-2-Methyl-2-propanesulfinamide solution 1?M in THF, contains BHT as inhibitor | 196929-78-9 | 50ML | ₹3896.9 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 497401 | (R)-(+)-2-Methyl-2-propanesulfinamide 98% | 196929-78-9 | 1G | ₹5564.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 497401 | (R)-(+)-2-Methyl-2-propanesulfinamide 98% | 196929-78-9 | 5G | ₹12665.25 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 497401 | (R)-(+)-2-Methyl-2-propanesulfinamide 98% | 196929-78-9 | 25G | ₹47597.53 | 2022-06-14 | Buy |
(R)-(+)-2-Methyl-2-propanesulfinamide Chemical Properties,Uses,Production
Chemical Properties
(R)-(+)-2-Methyl-2-propanesulfinamide is white to light yellow crystal powde
Uses
(R)-(+)-2-Methyl-2-propanesulfinamide is a chiral ligand, which is used in pharmaceutical compositions. Further, it is used in the preparation of beta-chloro sulfinamides in the synthesis of chiral azridines. It is involved in the preparation of organocatalyst for enantioselective reduction of imines. It serves as a reagent for synthesizing chiral amines. In addition to this, it is converted into P,N-sulfinyl imine ligands through condensation with aldehydes and ketones which undergoes iridium-catalyzed asymmetric hydrogenation of olefins.
Preparation
Acetic acid (70 g), R-tert-butylsulfinylhydrazine (42 g), zinc powder (60.5 g) and dichloromethane (150 mL) were added to the reaction flask. The temperature was slowly heated to 35-42 °C . After 16 hours, the filtrate was poured into 70 mL water. Dichloromethane (75 g×5) was added for extraction. Collected the organic phase, added 48% NaOH to adjust ρΗ (7-8). Then added NaCl, the layers were separated and the organic phase was washed with 15 g saturated aqueous solution of sodium chloride. The solution was dried over magnesium sulfate. After filterED, filtrate was concentrated under reduced pressure at 25-30 °C until no slipping. N-heptane was replaced, 28 g mixed solvent of N-heptane and toluene (N-heptane: toluene = 6:1) were added to perform beating at low temperature, filtered to obtain (R)-(+)-2-Methyl-2-propanesulfinamide. Yield=83%
References
[1] Qian X, et al. A stereoselective synthesis of (S)-2-(((3-fluoro-4-methylphenoxy)carbonyl)(1-(4-((5-methyl-2-phenyloxazol-4-yl)methoxy)phenyl)ethyl)amino)acetic acid, a highly potent PPAR α/γ dual agonist. Tetrahedron, 2015; 71: 9408-9414.
(R)-(+)-2-Methyl-2-propanesulfinamide Preparation Products And Raw materials
Raw materials
1of6
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JIVICHEM SYNTHESIS PVT LTD | +91-7028392790 +91-7028392790 | Maharashtra, India | 337 | 58 | Inquiry |
| Biosyn Research Chemicals | +91-91-9346022300 +91-9346022300 | Telangana, India | 465 | 58 | Inquiry |
| ssk bioscience pvt. ltd | +91-8106421818 +91-8106421818 | Hyderabad, India | 40 | 58 | Inquiry |
| Aravali Pharma And Lifesciences | +91-9350857917 +91-9810502945 | New Delhi, India | 119 | 58 | Inquiry |
| GLR Innovations | 08048961288 | Delhi, India | 94 | 58 | Inquiry |
| SL Drugs and Pharmaceuticals Pvt. Ltd. | +91 (40) 6661-1133 | New Delhi, India | 2688 | 50 | Inquiry |
| VIVAN Life Sciences Pvt. Limited. | +91(22) 2544 4584 / 2544 4585 | New Delhi, India | 138 | 50 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
Related articles
- Explore (R)-(+)-2-Methyl-2-propanesulfinamide: a key role in chiral chemical synthesis
- (R)-(+)-2-Methyl-2-propanesulfinamide has attracted extensive attention for its unique chemical properties and wide applicatio....
- Jul 2,2024
196929-78-9((R)-(+)-2-Methyl-2-propanesulfinamide)Related Search:
1of4
chevron_right




