Mecillinam
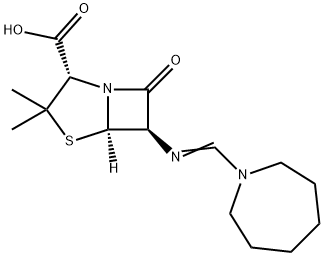
- CAS No.
- 32887-01-7
- Chemical Name:
- Mecillinam
- Synonyms
- AMDINOCILLIN;Coactin;Selexidin;Mecillinam (technical product);fl1060;Hexapen;FL10606;ro10-9070;MECILLINAM;Mecill·Nam
- CBNumber:
- CB7203891
- Molecular Formula:
- C15H23N3O3S
- Molecular Weight:
- 325.43
- MOL File:
- 32887-01-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:16
| Melting point | 156°C |
|---|---|
| Boiling point | 551℃ |
| alpha | D20 +285° (c = 1 in 0.1N HCl) |
| Density | 1.44±0.1 g/cm3(Predicted) |
| Flash point | >110°(230°F) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| pka | pKa 3.40 (Uncertain) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Soluble in water at approximately 1mg/ml |
| CAS DataBase Reference | 32887-01-7(CAS DataBase Reference) |
Mecillinam Chemical Properties,Uses,Production
Chemical Properties
White Solid
Uses
Mecillinam (amidinocillin) is a penicillin nucleus (6 APA) derivative, active in vitro against most aerobic and anaerobic Gram-negative bacilli, including E. coli and B. fraglis, but not active against Staphylococcus aureus, Enterococcus, or Pseudomonas. Mecillinam is synergistic with other beta-lactam drugs and therefore can be used in combination to treat severe Gram-negative infections. Although its in vitro efficacy is convincing, there are not enough clinical trials to support its use for intra-abdominal infections.
Antimicrobial activity
The antibacterial spectrum differs greatly from that of the
aminopenicillins in that the compound displays high activity
against many Gram-negative bacteria but limited activity
against Gram-positive organisms.
Mecillinam is active against many Enterobacteriaceae due to
its selective binding to PBP 2, although the susceptibility of
Proteus and Providencia spp. is variable. H. influenzae is less susceptible
than enteric bacilli, and Acinetobacter spp., B. fragilis
and Ps. aeruginosa are resistant.
It is readily inactivated by many β-lactamases, although it
is more stable than ampicillin.
Acquired resistance
Intrinsic resistance in susceptible species of enterobacteria is uncommon and many ampicillin-resistant strains are susceptible. Bacteria that are resistant to both ampicillin and mecillinam are usually those producing large amounts of β-lactamase, most commonly plasmid-mediated enzymes.
Pharmacokinetics
Oral absorption (pivmecillinam): c. 75%
Cmax 200 mg intravenous infusion: 12 mg/L end infusion
200 mg intramuscular: c. 6 mg/L after 45 min
400 mg oral (pivmecillinam): 2–5 mg/L after c. 1 h
Plasma half-life: 50 min
Volume of distribution: 0.2–0.4 L/kg
Plasma protein binding: 5–10%
Absorption
Oral absorption is very poor, with conventional doses producing plasma levels of <1 mg/L and recovery of only about 5% in the urine. A 400 mg dose of the pivaloyl ester is equivalent to 273 mg mecillinam. It is relatively well absorbed and rapidly liberates the parent compound. Metabolism and excretion
The amidino side chain undergoes spontaneous aqueous hydrolysis to the N-formyl derivative, which retains some antibacterial activity. Hydrolysis of the β-lactam ring also occurs.
Approximately 60% is excreted unchanged in the urine in the first 6 h, achieving concentrations exceeding 1 g/L. The concentration in bile can reach 40 or 50 mg/L in patients with normally functioning gallbladders treated with 800 mg intramuscularly.
Clinical Use
Urinary tract infection (pivmecillinam)
Other infections with susceptible Gram-negative bacilli (usually in
combination with other agents)
Side effects
It is generally well tolerated, and serious anaphylactic responses are said to be rare. Nausea and vomiting, which may be persistent, occur with diarrhea in some patients treated with pivmecillinam.
Mecillinam Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| VIVAN Life Sciences Pvt Ltd | +91-2225870080 +91-9987465183 | Maharashtra, India | 49 | 58 | Inquiry |
| Medec Dragon Private Limited | +91-9768474716 | Maharashtra, India | 59 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49392 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29825 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34571 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 8744 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
32887-01-7(Mecillinam)Related Search:
1of4
chevron_right




