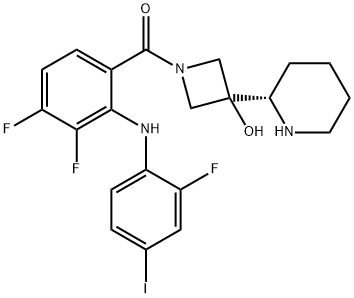
XL518
|
- ₹0
- Product name: XL518
- CAS: 934660-93-2
- MF: C21H21F3IN3O2
- MW: 531.31
- EINECS:
- MDL Number:MFCD22124461
- Synonyms:XL518;RG7420;[3,4-difluoro-2-[(2-fluoro-4-iodophenyl)aMino]phenyl][3-hydroxy-3-[(2S)-2-piperidinyl]-1-azetidinyl]Methanone;GDC-0973(XL-518);(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylaMino)phenyl)(3-hydroxy-3-(piperidin-2-yl)azetidin-1-yl)Methanone;XL518 ,GDC-0973;GDC-0973/RG7420;Methanone, [3,4-difluoro-2-[(2-fluoro-4-iodophenyl)aMino]phenyl][3-hydroxy-3-(2S)-2-piperidinyl-1-azetidinyl]-
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :165 - 166°C
Boiling point :565.9±50.0 °C(Predicted)
Density :1.706
storage temp. :Refrigerator
solubility :Chloroform (Slightly), DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly)
form :Solid
pka :13.13±0.20(Predicted)
color :Off-White
Boiling point :565.9±50.0 °C(Predicted)
Density :1.706
storage temp. :Refrigerator
solubility :Chloroform (Slightly), DMSO (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly)
form :Solid
pka :13.13±0.20(Predicted)
color :Off-White
Safety Information
| Symbol(GHS): |

|
|||||||
|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | |||||||
| Hazard statements: |
|
|||||||
| Precautionary statements: |
|
Description
Cobimetinib, codeveloped by Genentech and Exelixis, was approved in August 2015 in Switzerland and November 2015 in the U.S. and Europe for the treatment of unresectable or metastatic BRAFV600 mutationpositive melanoma when used in combination with vemurafenib. Cobimetinib is a potent, highly selective reversible inhibitor of mitogen-activated protein kinases (MEK) 1 and 2,120 which serves to inhibit phosphorylation of ERK1/2,121 disrupting the MAPK pathway which is responsible for cell proliferation, cell survival, and migration.122 Combination of cobimetinib with vemurafenib, an important BRAF inhibitor,123 enables targeting of multiple points on the MAPK pathway, leading to overall enhanced tumor cell apoptosis and response as compared to stand-alone treatment with vemurafenib.124 Specifically, in a representative trial of previously untreated patients with BRAFV600 mutation-positive, unresectable, stage IIIc or IV melanoma, combination of these two therapies led to a significantly improved progression-free survival and overall response rate versus patients treated only with vemurafenib.Related product price
- Ribonucleic acid
₹981-126099 - Capecitabine
₹5693.95-17320 - OTAVA-BB 1115529
₹10684.28-43061.85






