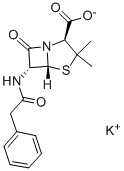
Penicillin G potassium salt
- CAS No.
- 113-98-4
- Chemical Name:
- Penicillin G potassium salt
- Synonyms
- PENICILLIN G POTASSIUM;BENZYLPENICILLIN POTASSIUM;PENICILLIN G POTASSIUM SALT;pfizerpen;Penicilline G;potassium [2s-(2alpha,5alpha,6beta)]-3,3-dimethyl-7-oxo-6-(phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate;Pen-G;forpen;PENICILLINE G POTASSIUM;R-Pen
- CBNumber:
- CB4855484
- Molecular Formula:
- C16H17KN2O4S
- Molecular Weight:
- 372.48
- MOL File:
- 113-98-4.mol
- Modify Date:
- 2025/6/12 13:02:17
| Melting point | 214-217 C |
|---|---|
| alpha | D22 +285° (c = 0.748 in water) |
| refractive index | 294 ° (C=1, H2O) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | H2O: 100 mg/mL |
| form | powder |
| color | Needles from butanol (aq) |
| PH | pH (10g/L, 25℃) : 5.0~7.5 |
| Water Solubility | Soluble in water (100 mg/ml), methanol, ethanol (sparingly), and alcohol. Insoluble in chloroform. |
| Merck | 14,7094 |
| BRN | 3832841 |
| Stability | Hygroscopic |
| InChIKey | IYNDLOXRXUOGIU-LQDWTQKMSA-M |
| SMILES | N12C([C@@H](NC(=O)CC3=CC=CC=C3)[C@@]1([H])SC(C)(C)[C@@H]2C([O-])=O)=O.[K+] |&1:2,13,19,r| |
| EPA Substance Registry System | Penicillin G Potassium (113-98-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317 | |||||||||
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P362+P364 | |||||||||
| Hazard Codes | Xn,C,F | |||||||||
| Risk Statements | 42/43-34-11 | |||||||||
| Safety Statements | 36/37-45-36/37/39-26-16-60-37-24-22 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | XH9700000 | |||||||||
| F | 10-23 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29411000 | |||||||||
| Toxicity | LD50 oral in rabbit: 5848mg/kg | |||||||||
| NFPA 704 |
|
Penicillin G potassium salt Chemical Properties,Uses,Production
Chemical Properties
Penicillin G potassium salt is also known as Potassium benzylpenicillin, it is white crystalline powder, odorless or slightly specific odor, hygroscopic. Soluble in water, physiological saline, glucose solution. The aqueous solution of it is easy to fail when placed at room temperature, and it will fail rapidly in the presence of acid, alkali, oxidant, etc. Penicillin G potassium salt has good antibacterial effect on Streptococcus such as Streptococcus hemolyticus, Streptococcus pneumoniae and Staphylococcus without penicillinase.
Uses
Penicillin G is a narrow spectrum antibiotic derived from Streptococcus pneumoniae. Penicillin G potassium salt is used as a cell culture additive as an antibiotics. Use to inhibit the synthesis of bacterial cell walls by inhibition of the cell wall peptidoglycan chain cross-lining. It is the drug of choice for groups A, B, C and G streptococci, nonenterococcal group D streptococci, viridians group streptococci, and non-penicillinase producing staphylococcus. The potassium salt has been used to study murosomes of staphylococci and the penicillin-induced lysis of Streptococcus mutans.
Preparation
Penicillin G potassium salt is a highly effective and low toxicity antibiotic, which is obtained by salt formation of Chlorpheniramine maleate and maleic acid.
Definition
ChEBI: Penicillin G potassium salt is organic potassium salt of benzylpenicillin. It is an antibiotic substance produced by Penicillium sp. Antibacterial. It contains a benzylpenicillin(1-).
General Description
Penicillin G Potassium is the potassium salt form of penicillin G, a broad-spectrum penicillin antibiotic. Penicillin G potassium binds to penicillin binding proteins (PBP), the enzymes that catalyze the synthesis of peptidoglycan, which is a critical component of the bacterial cell wall. This leads to the interruption of cell wall synthesis, consequently leading to bacterial cell growth inhibition and cell lysis.
Biochem/physiol Actions
Mode of Action: Penicillin G acts by inhibiting cell wall synthesis through binding to penicillin binding proteins (PBPs), inhibiting peptidoglycan chain cross-linking.
Antimicrobial spectrum: This product is active against gram-positive and gram-negative bacteria.
Safety Profile
Poison by intracerebral andintravenous routes. Moderately toxic by intraperitonealroute. Mutation data reported. See other penicillin entries.When heated to decomposition it emits toxic fumes ofNOx and SOx.
Penicillin G potassium salt Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Ebenezer Industries | Gujarat, India | 96 | 58 | Inquiry | |
| Pharmaffiliates Analytics & Synthetics (P) Ltd. | 91-172-5066494 | Haryana, India | 631 | 58 | Inquiry |
| Anika International Pvt. Limited | 91-11-47289900 | Delhi, India | 94 | 58 | Inquiry |
| Mesha Pharma Private Limited | 08048372569Ext 806 | Mumbai, India | 133 | 58 | Inquiry |
| Indian Commercial Company Pvt. Ltd. (ICC) | 91-22-22854565 | Maharashtra, India | 38 | 58 | Inquiry |
| bm chemie | 08048250160Ext 924 | Mumbai, India | 82 | 58 | Inquiry |
| Maas Pharma Chemicals | +91-9958666224 | Delhi, India | 1607 | 58 | Inquiry |
| Hindchem Corporation | 08048976759 | Mumbai, India | 118 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Biopharmacule Speciality Chemicals P Ltd (India) | +91 40 6462 1866 | Hyderabad, India | 526 | 58 | Inquiry |
Related articles
- Penicillin G potassium salt: a normal form of penicillin
- Penicillin G potassium salt is a widely studied form of penicillin used extensively in microbiological research for its antiba....
- Mar 11,2025
113-98-4(Penicillin G potassium salt)Related Search:
1of4
chevron_right




