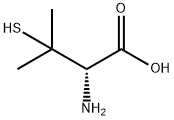
D-Penicillamine
- CAS No.
- 52-67-5
- Chemical Name:
- D-Penicillamine
- Synonyms
- PENICILLAMINE;PENICILLAMINE;PENICILLAMINE;Penicillamin;2-Amino-3-mercapto-3-methylbutanoic acid;usan;Depen;Cuprimine;H-D-PEN-OH;Pencillamine
- CBNumber:
- CB5742367
- Molecular Formula:
- C5H11NO2S
- Molecular Weight:
- 149.21
- MOL File:
- 52-67-5.mol
- Modify Date:
- 2024/6/23 21:00:51
| Melting point | 210 °C (dec.)(lit.) |
|---|---|
| Boiling point | 251.8±35.0 °C(Predicted) |
| alpha | -65 º (c=5, 1M NaOH, on dry) |
| Density | 1.113 (estimate) |
| refractive index | -63 ° (C=1, 1mol/L NaOH) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble100mg/mL |
| form | Powder |
| pka | pKa 7.83±0.01(H2O,t =37±0.05,I=0.15)(Approximate) |
| color | White to almost white |
| Water Solubility | 11.1 g/100 mL (20 ºC) |
| Merck | 14,7088 |
| BRN | 1722375 |
| BCS Class | 3 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | VVNCNSJFMMFHPL-VKHMYHEASA-N |
| CAS DataBase Reference | 52-67-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Penicillamine(52-67-5) |
| EPA Substance Registry System | Penicillamine (52-67-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361d | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 | |||||||||
| Hazard Codes | Xi,T,Xn | |||||||||
| Risk Statements | 36/37/38-40-20/21/22 | |||||||||
| Safety Statements | 26-36-24/25-22 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | YV9425000 | |||||||||
| Hazard Note | Toxic | |||||||||
| HS Code | 29309016 | |||||||||
| Toxicity | LD50 in rats (mg/kg): >10000 orally, >660 i.p. (Jaffe) | |||||||||
| NFPA 704 |
|
D-Penicillamine price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | P4875 | D-Penicillamine 98-101% | 52-67-5 | 1G | ₹4200.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | P4875 | D-Penicillamine 98-101% | 52-67-5 | 5G | ₹12892.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR2638 | Penicillamine Pharmaceutical Secondary Standard; Certified Reference Material | 52-67-5 | 500MG | ₹20415.95 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | P4875 | D-Penicillamine 98-101% | 52-67-5 | 25G | ₹28166.65 | 2022-06-14 | Buy |
| TCI Chemicals (India) | P0147 | D-Penicillamine | 52-67-5 | 5G | ₹4600 | 2022-05-26 | Buy |
D-Penicillamine Chemical Properties,Uses,Production
Description
Penicillamine is an orally bioavailable copper chelator and penicillin degradation product. It increases urinary and fecal copper excretion and decreases liver copper concentration in a rat model of copper overload when administered at 0.67 mmol/kg per day, but does not affect kidney, spleen, or brain copper levels. Penicillamine (100 mg/kg per day) dissolves copper-rich granules in hepatic lysosomes of Long-Evans cinnamon (LEC) rats, which spontaneously develop hepatic injury and acute hepatitis and have a mutation homologous to that of the human Wilson disease gene. Penicillamine has anticonvulsant and proconvulsant effects in mice when administered at 0.5 and 250 mg/kg, respectively, which are blocked by the nitric oxide synthase (NOS) inhibitors L-NAME and 7-nitroindazole . Formulations containing penicillamine have been used to treat Wilson disease, cystinuria, and active rheumatoid arthritis.
Chemical Properties
White to off-white crystalline powder
Uses
As a Penicillin metabolite, D-(-)-Penicillamin can be used in the treatment of Wilson’s disease, Cystinuria, Scleroderma and arsenic poisoning.
Definition
ChEBI: An optically active form of penicillamine having D-configuration. Pharmaceutical form (L-form is toxic) of chelating agent used to treat heavy metal poisoning.
brand name
Cuprimine(Merck); Depen (Medpointe), Artamin (Sanabo, Austria), Atamir (Sandoz, Denmark), Dimetylcystein (Lilly, Denmark), Metalcaptase (Knoll, South Africa), Reumacillin (Leiras, Finland), Trisorcin (Merckle, Germany), Trolovol (Asta Medica, Germany).
General Description
D-Penicillamine contains a β-lactam chemical structure.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by subcutaneous and intravenous routes. Mildly toxic by ingestion. An experimental teratogen. Human systemic effects by ingestion: agranulocytosis, dermatitis, fever, hemorrhage, increased body temperature, dermatitis, leukopenia, proteinuria, thrombocytopenia. Human teratogenic effects by an unspecified route: developmental abnormalities of the craniofacial areas, skin, and skin appendages, and body wall. Experimental reproductive effects. Questionable human carcinogen producing leukemia. Mutation data reported. Used in the treatment of rheumatoid arthritis, metal poisonings, and cystinuria. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also MERCAPTANS.
Synthesis
d-penicillamine can be synthesized in a multistep process that begins with
heating isobutyraldehyde, pyridine, sulfur, and
ammonia in benzene to form 5,5-dimethyl-2-
isopropyl-?3-thiazoline. Treatment with hydrogen cyanide gives 4-cyano-5,5-dimethyl-
2-isopropylthiazolidine, which on acid hydrolysis gives d,l-penicillamine hydrochloride. Resolution is accomplished by
conversion of the racemate to d,l-3-formyl-
2,2,5,5-tetramethylthiazolidine-4-carboxylic
acid by treatment first with acetone, then
with acetic formic anhydride. The enantiomers are separated in the usual manner,
using, for example, l-lysine or d-(?)-
threo-1-(4-nitrophenyl)-2-aminopropane-1,3-
diol. Acidification liberates d-3-formyl-
2,2,5,5-tetramethylthiazolidine-4-carboxylic
acid, which is hydrolyzed with hydrochloric
acid to yield d-penicillamine hydrochloride.
Neutralization with ethanolic triethylamine affords d-penicillamine.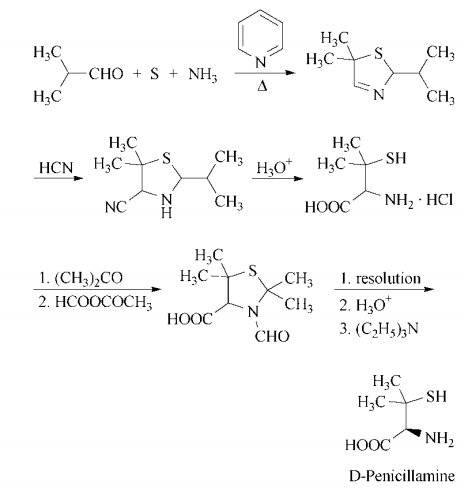
Purification Methods
The melting point of D-(-)-penicillamine depends on the rate of heating (m 202-206o is obtained by starting at 195o and heating at 2o/minute). It is soluble in H2O and alcohols but insoluble in Et2O, CHCl3, CCl4 and hydrocarbon solvents. Purify it by dissolving it in MeOH and adding Et2O slowly. Dry it in vacuo and store it under N2. [Weight et al. Angew Chem, Int Ed (English) 14 330 1975, Cornforth in The Chemistry of Penicillin (Clarke, Johnson and Robinson eds) Princeton Univ Press, 455 1949, Review: Chain et al. Antibiotics (Oxford University Press) 2 1949, Polymorphism: Vidler J Pharm Pharmacol 28 663 1976]. The D-S-benzyl derivative has m 197-198o (from H2O), [] D 17 -20o (c 1, N NaOH), -70o (N HCl). [Beilstein 4 IV 3228.]
D-Penicillamine Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Prajna generics pvt ltd | +91-9441174873 +91-9441174873 | Hyderabad, India | 24 | 58 | Inquiry |
| Varanous Labs Pvt Ltd | +91-7036248882 +91-7036248882 | Hyderabad, India | 1541 | 58 | Inquiry |
| Sekhment pharmaventures | +91-9030088669 +91-9030088669 | Mumbai, India | 105 | 58 | Inquiry |
| Apothecon Pharmaceuticals Pvt Ltd | +91-2662244212 +91-9099007601 | Gujarat, India | 28 | 58 | Inquiry |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | +91-26463395 +91-7926462688 | Gujarat, India | 1615 | 58 | Inquiry |
| Fleming Laboratories Ltd | +91-9666407333 +91-9666022445 | Hyderabad, India | 22 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Biophore India Pharmaceuticals Pvt Ltd | +91-9100031592 +91-9030907714 | Telangana, India | 134 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
Related articles
- Synthesis and Application of D-Penicillamine
- D-Penicillamine (1) is a colorless crystalline powder with a weak odor typical of sulfur-containing amino acids, and a charact....
- May 24,2022





