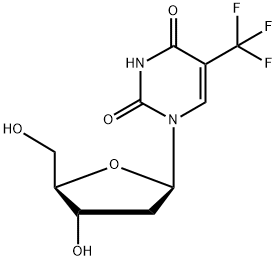
Trifluridine
- CAS No.
- 70-00-8
- Chemical Name:
- Trifluridine
- Synonyms
- TFT;TRIFLUOROTHYMIDINE;viroptic;f3t;nsc75520;Triflurdine;Triflurdine (Viroptic);Thymidine, α,α,α-trifluoro-;2,4(1h,3h)-pyrimidinedione,1-(2-deoxy-beta-d-ribofuranosyl)-5-(trifluorometh;FTD
- CBNumber:
- CB6768812
- Molecular Formula:
- C10H11F3N2O5
- Molecular Weight:
- 296.2
- MOL File:
- 70-00-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 18:29:10
| Melting point | 190-193 °C (lit.) |
|---|---|
| Density | 1.4365 (estimate) |
| refractive index | 50 ° (C=1, H2O) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Water (up to 14 mg/ml) |
| form | solid |
| pka | pKa 7.85 (Uncertain) |
| color | Off-white |
| Merck | 14,9687 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 70-00-8(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H341-H351-H361d | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 20/21/22-40 | |||||||||
| Safety Statements | 22-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | XP2087500 | |||||||||
| Hazard Note | Keep Cold | |||||||||
| HS Code | 29349990 | |||||||||
| Toxicity | LD50 intraperitoneal in mouse: 1931mg/kg | |||||||||
| NFPA 704 |
|
Trifluridine price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | T2255 | Trifluorothymidine ≥99% (HPLC) | 70-00-8 | 100MG | ₹26856.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T2255 | Trifluorothymidine ≥99% (HPLC) | 70-00-8 | 1G | ₹169616.93 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR3150 | Trifluridine pharmaceutical secondary standard, certified reference material | 70-00-8 | 200MG | ₹27040.85 | 2022-06-14 | Buy |
| TCI Chemicals (India) | T2511 | Trifluorothymidine | 70-00-8 | 100MG | ₹9200 | 2022-05-26 | Buy |
| TCI Chemicals (India) | T2511 | Trifluorothymidine | 70-00-8 | 1G | ₹37500 | 2022-05-26 | Buy |
Trifluridine Chemical Properties,Uses,Production
Description
Trifluridine (trifluorothymidine, TFT), a fluorinated pyrimidine nucleoside, is an anti-herpesvirus agent and an antitumor antimetabolite agent. It is an analog of thymidine which inhibits thymidylate synthase possesses antiviral and anticancer activity. After phosphorylation by thymidine kinase, it is incorporated into DNA where it induces DNA-damage and interferes with repair enzymes. Enhances frame shift insertion and deletion in CRISPR genome editing in pluripotent stem cells.
Chemical Properties
White to Off-White Solid
Uses
Trifluridine is used as anti-herpesvirus antiviral agent in ophthalmie preparations.
Indications
Trifluridine (Viroptic) is a fluorinated pyrimidine nucleoside that has in vitro activity against HSV-1 and HSV- 2, vaccinia, and to a lesser extent, some adenoviruses. Activation of trifluridine requires its conversion to the 5 monophosphate form by cellular enzymes.Trifluridine monophosphate inhibits the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) by thymidylate synthetase. In addition, it competes with deoxythymidine triphosphate (dTTP) for incorporation by both viral and cellular DNA polymerases. Trifluridine-resistant mutants have been found to have alterations in thymidylate synthetase specificity.
Definition
ChEBI: Trifluridine is a pyrimidine 2'-deoxyribonucleoside compound having 5-trifluoromethyluracil as the nucleobase. An antiviral drug used mainly in the treatment of primary keratoconjunctivitis and recurrent epithelial keratitis. It has a role as an antiviral drug, an antimetabolite, an EC 2.1.1.45 (thymidylate synthase) inhibitor and an antineoplastic agent. It is a nucleoside analogue, an organofluorine compound and a pyrimidine 2'-deoxyribonucleoside.
General Description
Trifluridine, 5-trifluoromethyl-29-deoxyuridine (Viroptic),is a fluorinated pyrimidine nucleoside that demonstrates invitro inhibitory activity against HSV-1 and HSV-2, CMV,vaccinia, and some adenoviruses.Trifluridine possesses atrifluoromethyl group instead of an iodine atom at the 5-position of the pyrimidine ring.
synthetase, and the biologically generated triphosphatecompetitively inhibits thymidine triphosphate incorporationinto DNA by DNA polymerase. In addition, trifluridine inits triphosphate form is incorporated into viral and cellularDNA, creating fragile, poorly functioning DNA.Trifluridine is approved in the United States for the treatmentof primary keratoconjunctivitis and recurrent epithelialkeratitis caused by HSV types 1 and 2. Topical trifluridineshows some efficacy in patients with acyclovir-resistantHSV cutaneous infections.
Mechanism of action
Trifluorothymidine is a fluorinated pyridine nucleoside structurally related to idoxuridine. It has been approved by the U.S. FDA and is a potent, specific inhibitor of replication of HSV-1 in vitro. Its mechanism of action is similar to that of idoxuridine. Like other antiherpes drugs, it is first phos-phorylated by thymidine kinase to mono-, di-, and triphosphate forms, which are then incorporated into viral DNA in place of thymidine to stop the formation of late virus mRNA and subsequent synthesis of the virion proteins.
Pharmacokinetics
Trifluorothymidine is a synthetic halogenated pyrimidine nucleoside, first synthesized as an antitumor agent. It inhibits enzymes of the DNA pathway and is incorporated into both cellular and progeny viral DNA, causing faulty transcription of late messenger RNA and the production of incompetent virion protein. It does not require a viral thymidine kinase for monophosphorylation and is far less selective and more toxic than other analogs. It is active against HSV-1 and HSV-2, vaccinia virus, CMV and possibly adenovirus. Trifluorothymidine, when given IV, shows a plasma half-life of 18 minutes and is excreted in the urine either unchanged or as the inactive metabolite 5-carboxyuracil. When applied as a 1% ophthalmic solution, it rapidly enters the aqueous humor of HSV-infected rabbits’ eyes but is cleared within 60–90 min.
Side effects
The most frequent adverse reactions to trifluridine administration
are transient burning or stinging and
palpebral edema. Other adverse reactions include superficial
punctate keratopathy, epithelial keratopathy,
hypersensitivity, stromal edema, irritation, keratitis
sicca, hyperemia, and increased intraocular pressure.
Trifluridine is mutagenic in vitro and carcinogenic
and teratogenic when administered subcutaneously to
animals. Topical trifluridine was not teratogenic in animal
studies. Because it is applied topically in humans,
the likelihood of systemic effects is low.
Trifluridine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Biophore India Pharmaceuticals Pvt Ltd | +91-9100031592 +91-9030907714 | Telangana, India | 134 | 58 | Inquiry |
| Mylan Laboratories Ltd | +91-4023550543 +91-4030866666 | Telangana, India | 150 | 58 | Inquiry |
| Apicore Pharmaceuticals Pvt Ltd | +91-2662267177 +91-2662267166 | Gujarat, India | 181 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| BIOPHORE INDIA PHARMACEUTICALS PVT LTD | +91-4047474545 | New Delhi, India | 105 | 58 | Inquiry |
Related articles
- Trifluorothymidine/Trifluoridine
- Trifluorothymidine, also known as trifluoridine, is a fluorinated pyrimidine nucleoside analog of thymidine and related to ido....
- Apr 13,2022
70-00-8(Trifluridine)Related Search:
1of4
chevron_right




