Losartan
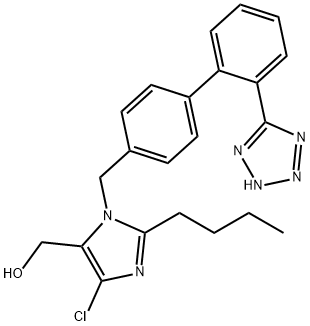
- CAS No.
- 114798-26-4
- Chemical Name:
- Losartan
- Synonyms
- LOS;dup89;LOSARTAN;Compound 89;Losartan(base);Losartan (DUP 89);nyl)-4-yl)methyl)-;Losartan (DuP-753);Losartan USP/EP/BP;Losartan - Bio-X ?
- CBNumber:
- CB8120081
- Molecular Formula:
- C22H23ClN6O
- Molecular Weight:
- 422.91
- MOL File:
- 114798-26-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 183-184 C |
|---|---|
| Boiling point | 682.0±65.0 °C(Predicted) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| vapor pressure | 0.002Pa at 20℃ |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 5-6(at 25℃) |
| color | White to Off-White |
| Water Solubility | 4.8mg/L at 20℃ |
| LogP | 1.1 at 20℃ |
| CAS DataBase Reference | 114798-26-4(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Imidazole-5-methanol, 2-butyl-4-chloro-1-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]- (114798-26-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H360FD-H362 |
| Precautionary statements | P202-P260-P263-P280-P302+P352-P308+P313 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| Hazardous Substances Data | 114798-26-4(Hazardous Substances Data) |
Losartan Chemical Properties,Uses,Production
Uses
Losartan is a nonpeptide angiotensin II AT1-receptor antagonist. Antihypertensive.
Definition
ChEBI: A biphenylyltetrazole where a 1,1'-biphenyl group is attached at the 5-position and has an additional trisubstituted imidazol-1-ylmethyl group at the 4'-position
General Description
Losartan, 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-yl-phenyl)benzyl]imidazole-5-methanol monopotassiumsalt (Cozarr), was the first nonpeptide imidazole to beintroduced as an orally active angiotensin II antagonist withhigh specificity for AT1. When administered to patients, itundergoes extensive first-pass metabolism, with the 5-methanol being oxidized to a carboxylic acid. This metabolismis mediated by CYP 2C9 and 3A4 isozymes. The 5-methanol metabolite is approximately 15 times more potentthan the parent hydroxyl compound. Because the parent hydroxylcompound has affinity for the AT1 receptor, strictlyspeaking, it is not a prodrug.
Losartan Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Vijayasri Organics Private Limited | +91-9848990113 +91-9848990113 | Telangana, India | 43 | 58 | Inquiry |
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 159 | 58 | Inquiry |
| Viyash Life Sciences Pvt Ltd | +91-4023635052 +91-4023635052 | Telangana, India | 213 | 58 | Inquiry |
| Sainor Life Sciences Pvt Ltd | +919542990009 | AndhraPradesh, India | 13 | 58 | Inquiry |
| Divis Laboratories Ltd | +91-4066966413 +91-4066966413 | Hyderabad, India | 34 | 58 | Inquiry |
| Lakshmi Farmachem | +91-9948795885 +91-9550886476 | Telangana, India | 407 | 58 | Inquiry |
| PSN Medicare Private Limited | +91-9030090313 +91-7702388996 | Telangana, India | 82 | 58 | Inquiry |
| Chempifine Chemicals | +91-2225667766 +91-2225667766 | Punjab, India | 144 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Lavybens Pharma | +91-8790466757 +91-8790466756 | Telangana, India | 80 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Vijayasri Organics Private Limited | 58 |
| J S LABS | 58 |
| Viyash Life Sciences Pvt Ltd | 58 |
| Sainor Life Sciences Pvt Ltd | 58 |
| Divis Laboratories Ltd | 58 |
| Lakshmi Farmachem | 58 |
| PSN Medicare Private Limited | 58 |
| Chempifine Chemicals | 58 |
| Ralington Pharma | 58 |
| Lavybens Pharma | 58 |
114798-26-4(Losartan)Related Search:
1of4
chevron_right




