Abacavir sulfate
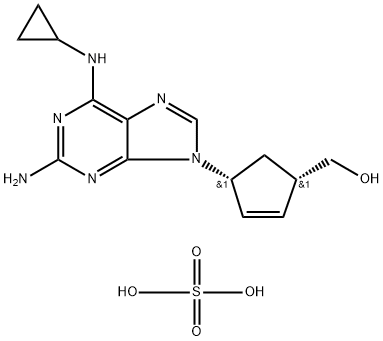
- CAS No.
- 188062-50-2
- Chemical Name:
- Abacavir sulfate
- Synonyms
- ABACAVIR SULPHATE;ZIAGEN;DRG 0257;Hsdb 7154;ABC sulfate;1592U89 sulfate;Unii-J220T4J9Q2;bacavir sulfate;ABACAVIR SULFATE;Abacavir Hemisulfate
- CBNumber:
- CB8425548
- Molecular Formula:
- C14H20N6O5S
- Molecular Weight:
- 384.41
- MOL File:
- 188062-50-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/4/16 18:29:06
| Melting point | 222-225°C |
|---|---|
| storage temp. | 2-8°C |
| solubility | H2O: ≥17mg/mL |
| form | powder |
| color | white to tan |
| optical activity | [α]/D -30 to -40°, c = 0.5 in methanol |
| Water Solubility | 1.68ug/L(32 ºC) |
| BCS Class | 3 |
| CAS DataBase Reference | 188062-50-2(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317-H319-H341-H351-H361 |
| Precautionary statements | P201-P202-P280-P302+P352-P305+P351+P338-P308+P313 |
| Hazard Codes | Xn |
| Risk Statements | 63-40 |
| Safety Statements | 36/37 |
| WGK Germany | 3 |
| HS Code | 2933595960 |
Abacavir sulfate price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML0089 | Abacavir sulfate ≥98% (HPLC) | 188062-50-2 | 10MG | ₹11499.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML0089 | Abacavir sulfate ≥98% (HPLC) | 188062-50-2 | 50MG | ₹46509 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1256 | Abacavir sulfate Pharmaceutical Secondary Standard; Certified Reference Material | 188062-50-2 | 500MG | ₹22516 | 2022-06-14 | Buy |
Abacavir sulfate Chemical Properties,Uses,Production
Description
Abacavir sulfate was first launched as Ziagen in the US for the treatment of human immunodeficiency virus (HIV) infection, in combination with other antiretroviral drugs. Abacavir is a carbocyclic nucleoside reverse transcriptase inhibitor (nRTI); it is one of the most potent anti-HIV agents to date. In vitro, Abacavir is a potent and selective inhibitor of HIV-1 and HIV-2 replication. Resistance to Abacavir develops more slowly than for other anti-HIV agents. Abacavir is highly synergistic with protease inhibitors such as Amprenavir. In clinical trials for HIV infections in adults, it produced durable suppression in viral load. Combinations with different protease inhibitors such as Nelfinavir, Saquinavir or Indinavir markedly reduced plasma viral load to undetectable levels for at least 48 weeks, and significantly raised CD4+ cell counts in adults with HIV infection, especially nRTI-naive patients. Abacavir has a good oral availability and its penetration into CSF is much more significant than for other anti-HIV drugs. The two major metabolites identified in humans were the 5'-carboxylate and the 5'-glucuronide, mainly excreted via the renal route.
Chemical Properties
Abacavir sulfate is white to off-white crystalline powder and the solubility is pH dependent with minimal solubility at basic pH and increased solubility at acid. This active substance is slightly soluble in diethyl ether and ethanol. Abacavir exhibits stereoisomerism due to the presence of two chiral centres (1S,4R absolute configuration).
Uses
Abacavir sulfate is a nucleoside reverse transcriptase inhibitor (NRTI) used for the treatment of HIV-1 infection and anti-AIDS drug. It has been used in the cytotoxicity to test its tumour promoting activity U937 cells.
Definition
ChEBI: Abacavir sulfate is an azaheterocycle sulfate salt that is the sulfate salt of the HIV-1 reverse transcriptase inhibitor abacavir. It is functionally related to an abacavir.
General Description
Abacavir sulfate belongs to the class of human immunodeficiency virus (HIV) medicines called nucleoside reverse transcriptase inhibitors, with antiretroviral activity against HIV.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Side effects
Abacavir can cause serious, life-threatening side effects. These include allergic reactions, a buildup of lactic acid in the blood (lactic acidosis), and liver problems. People who take abacavir may have a serious allergic reaction (hypersensitivity reaction) that can cause death.
Common side effects of Ziagen include: trouble sleeping, loss of appetite, strange dreams, headache, ear pain, cold symptoms (stuffy nose, sneezing, sinus pain), or changes in the shape or location of body fat (especially in your arms, legs, face, neck, breasts, and trunk).
Synthesis
Abacavir Sulfate can be prepared by an enantioselective synthesis involving palladium-catalyzed coupling of a chloropurine with a carbocyclic allylic diacetate.
Synthesis Step: Treatment of 2,5-diamino-4,6-dihydroxypyrimidine (I) with (chloromethylene)dimethylammonium chloride yielded the dichloropyrimidine with both amino groups derivatized as amidines. Partial hydrolysis with aqueous HCl in hot ethanol gave N-(2-amino-4,6-dichloro-pyrimidin-5-yl)-N,Ndimethylformamidene (II). Subseqent buffered hydrolysis at pH 3.2 yielded the (2-amino-4,6-dichloro-pyrimididin-5-ylamino)acetaldehyde (III). Condensation chloropyrimidine (III) with (1S,4R)-4-amino-2-cyclopentene-1- methanol (IV) in the presence of triethylamine and NaOH gave [2-amino-4- chloro-6-(4-hydroxymethyl-cyclopent-2-enylamino)pyrimidin-5-ylamino]- acetaldehyde (V). The correct enantiomer (IV) of racemic aminocyclopentene was obtained by resolution of diastereomeric salts with D-dibenzoyltartaric acid. Cyclization of (V) to the corresponding purine was accomplished with refluxing triethyl orthoformate or diethoxymethyl acetate to give nucleoside analogue [4-(2-amino-6-chloro-purin-9-yl)-cyclopent-2-enyl]methanol (VI). Displacement of chloride in the purine nucleus with cyclopropyl amine in refluxing butanol afforded abacavir. The structure of obtained compound was confirmed by 1H NMR method and elemental analysis.
In practice it is usually used as sulfate salt.
Solubility in organics
Soluble in water (77 mg/ml), DMSO (< 1 mg/ml at 25° C), ethanol (< 1 mg/ml at 25° C), and methanol.
Abacavir sulfate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CDYMAX | +91-9019317788 +91-9019317788 | Bangalore, India | 28 | 58 | Inquiry |
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| Styrax Pharma Pvt Ltd | +91-4048583333 +91-9848912103 | Telangana, India | 24 | 58 | Inquiry |
| Aurobindo Pharma Limited | +914066725000 | Telangana, India | 112 | 58 | Inquiry |
| Apotex Pharmachem India Pvt Ltd | +91-8022891034 +91-8022891000 | Karnataka, India | 109 | 58 | Inquiry |
| Lupin Ltd | +91-8019896181 +91-8019896181 | Maharashtra, India | 93 | 58 | Inquiry |
| Cipla Ltd | +912224826000 | Maharashtra, India | 133 | 58 | Inquiry |
| Raising Sun Pharma | +91-9399941155 +91-9399941155 | Telangana, India | 127 | 58 | Inquiry |
| Arene Lifesciences Limited | +91-9849847907 +91-8455241148 | Telangana, India | 55 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| CDYMAX | 58 |
| Gonane Pharma | 58 |
| Styrax Pharma Pvt Ltd | 58 |
| Aurobindo Pharma Limited | 58 |
| Apotex Pharmachem India Pvt Ltd | 58 |
| Lupin Ltd | 58 |
| Cipla Ltd | 58 |
| Raising Sun Pharma | 58 |
| Arene Lifesciences Limited | 58 |
| Ralington Pharma | 58 |
188062-50-2(Abacavir sulfate)Related Search:
1of4
chevron_right




