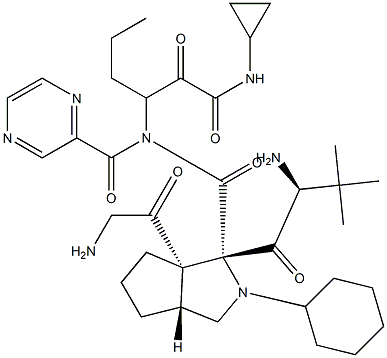
Telaprevir
|
- ₹6007.88 - ₹24345.43
- Product name: Telaprevir
- CAS: 402957-28-2
- MF: C36H53N7O6
- MW: 679.85
- EINECS:609-814-6
- MDL Number:MFCD11616089
- Synonyms:(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxaMido)acetaMido)-3,3-diMethylbutanoyl)-N-((S)-1-(cyclopropylaMino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxaMide;Cyclopenta(c)pyrrole-1-carboxamide, (2S)-2-cyclohexyl-N-(pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-((1S)-1-((cyclopropylamino)oxoacetyl)butyl)octahydro-, (1S,3aR,6aS)-;VX-950;VX-950 (Teleprevir);(1S,3aR,6aS)-(2S)-2-Cyclohexyl-N-(pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-((1S)-1-((cyclopropylamino)oxoacetyl)butyl)octahydrocyclopenta[c]pyrrole-1-carboxamide;Telaprevir;(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-6-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)-octahydrocyclopenta[c]pyrrole-1-carboxamide VX-950;TELAPREVIR; VX-950
2 prices
Selected condition:
Brand
- Sigma-Aldrich(India)
Package
- 5MG
- 25MG
- ManufacturerSigma-Aldrich(India)
- Product numberSML2654
- Product descriptionTelaprevir ≥90% (HPLC)
- Packaging5MG
- Price₹6007.88
- Updated2022-06-14
- Buy
- ManufacturerSigma-Aldrich(India)
- Product numberSML2654
- Product descriptionTelaprevir ≥90% (HPLC)
- Packaging25MG
- Price₹24345.43
- Updated2022-06-14
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML2654 | Telaprevir ≥90% (HPLC) | 5MG | ₹6007.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML2654 | Telaprevir ≥90% (HPLC) | 25MG | ₹24345.43 | 2022-06-14 | Buy |
Properties
Melting point :116-123°C
Density :1.25
storage temp. :Sealed in dry,Store in freezer, under -20°C
solubility :Chloroform, Methanol (Slightly)
pka :11.84±0.20(Predicted)
form :Solid
color :Pale Yellow to Light Yellow
Density :1.25
storage temp. :Sealed in dry,Store in freezer, under -20°C
solubility :Chloroform, Methanol (Slightly)
pka :11.84±0.20(Predicted)
form :Solid
color :Pale Yellow to Light Yellow
Safety Information
| Symbol(GHS): |

|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||
| Hazard statements: |
|
||||||||||||
| Precautionary statements: |
|
Description
The hepatitis C virus (HCV) protease inhibitor telaprevir (VX-950, MP- 424,LY-570310) was approved by the U.S. FDA in May 2011 for the treatment of genotype 1 chronic HCV infection in adult patients in combination with peginterferon alfa and ribavirin (PR). Telaprevir and boceprevir (vide supra) are the first two HCV protease inhibitors to be approved for treatment of HCV infection. Telaprevir is a HCV NS3-4A protease inhibitor that exerts its antiviral effect by blocking the release of nonstructural viral proteins from a polyprotein precursor. Telaprevir is a potent inhibitor of the protease (IC50=10 nM) and is active in cell culture (HCV 1b replicon assay, EC50=354 nM). Telaprevir was identified from efforts to truncate a decamer peptide inhibitor derived from the natural substrate NS5A-5B and was guided by structure-based design. The ketoamide group of telaprevir forms a covalent, reversible bond with the active site serine hydroxyl of the protease and compensates for the loss of affinity resulting from truncation of the peptide. Despite the presence of the reactive keto-amide group, telaprevir is >500-fold less potent against other serine proteases. Synthesis of the key octahydrocyclopenta[c]pyrrole-1-carboxylic acid fragment of telaprevir is achieved by a-deprotonation of Boc-protected 3-azabicyclo[3.3.0]nonane followed by reaction with CO2 and resolution of the racemic acid. Alternatively, deprotonation is carried out in the presence of a chiral amine to give the enantiomerically enriched acid.More related product prices
918504-65-1 Carfilzomib 2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benimidazole-4- carboxamide dimethyl (2S,2'S)-1,1'-((2S,2'S)-2,2'-(4,4'-(biphenyl-4,4'-diyl)bis(1H-imidazole-4,2-diyl))bis(pyrrolidine-2,1-diyl))bis(3-methyl-1-oxobutane-2,1-diyl)dicarbamate Bortezomib AC480 (BMS-599626) Unii-U2Y5ofn795 CUDC-101 RO4929097 Avagacestat (BMS-708163) 1222998-36-8 958852-01-2 1072833-77-2 Tariquidar CCT 137690 VX 702 RG7227, ITMN-191, RO5190591 Tozasertib VX-745 PimodivirRelated product price
- Bortezomib
₹12940 - AC480 (BMS-599626)
₹7700-22000 - CUDC-101
₹19095.3-71445
Suppliers and manufacturers
BDR Pharmaceuticals International Pvt Ltd
GLENMARK LIFE SCIENCES LTD
CLEARSYNTH LABS LTD.
Pharmaffiliates Analytics and Synthetics P. Ltd
Chiral Biosciences Limited
Pharma Affiliates
Chiral BioSciences Ltd
BDR Pharmaceuticals International Pvt., Ltd.
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
Global Chem Asia Pacific






