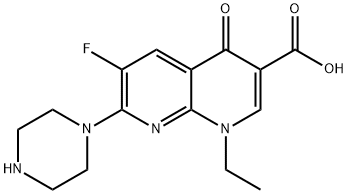
Enoxacin
- CAS No.
- 74011-58-8
- Chemical Name:
- Enoxacin
- Synonyms
- Penetrex;1-ETHYL-6-FLUORO-1,4-DIHYDRO-4-OXO-7-[1-PIPERAZINYL]-1,8-NAPHTHYRIDINE-3-CARBOXYLIC ACID;ENX;ci919;enoram;Abenox;Enoxen;Enoxor;Humark;at-2266
- CBNumber:
- CB2403021
- Molecular Formula:
- C15H17FN4O3
- Molecular Weight:
- 320.32
- MOL File:
- 74011-58-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/11/17 16:00:36
| Melting point | 220-224 C |
|---|---|
| Boiling point | 569.9±50.0 °C(Predicted) |
| Density | 1.2931 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 6.04±0.70(Predicted) |
| form | Solid |
| color | Off-White to Pale Yellow |
| Water Solubility | 50 mg/ml in 1 M NaOHSlightly soluble in sodium hydroxide, dimethyl sulfoxide, chloroform and methanol. Insoluble in water. |
| CAS DataBase Reference | 74011-58-8(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H320-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36/37 |
| WGK Germany | 2 |
| RTECS | QN2800000 |
| HS Code | 29335995 |
| Toxicity | LD50 in male, female mice, male, female rats (mg/kg): 327, 391, 236, 294 i.v.; 1237, 1320, >2000, >2000 s.c.; all >5000 orally (Senda) |
Enoxacin Chemical Properties,Uses,Production
Description
Enoxacin is a broad spectrum, quinolone-class, antibacterial agent closely related structurally to nalidixic acid. The serum half-life (6.2 hrs.) and urinary recovery (70%) are considerably greater than for other newer agents of this class, such as norfloxacin (10) and earlier mentioned ciprofloxacin.
Chemical Properties
Off-White to Pale Yellow Solid
Uses
Enoxacin is an antibacterial agent used in the treatment of gastro enteritis including infectious diarrhea, respiratory tract infections, gonorrhea and urinary tract infections. It is a bactericidal and its mode of action depends on blocking of bacterial DNA replication by binding itself to an enzyme called DNA gyrase.
Definition
ChEBI: A 1,8-naphthyridine derivative that is 1,4-dihydro-1,8-naphthyridine with an ethyl group at the 1 position, a carboxy group at the 3-position, an oxo sustituent at the 4-position, a fluoro substituent at the 5-position and a piperazin-1-yl group at the 7 p sition. An antibacterial, it is used in the treatment of urinary-tract infections and gonorrhoea.
Pharmaceutical Applications
A 1,8 naphthyridone derivative available as an oral drug. It exhibits good activity in vitro against many species of Enterobacteriaceae. It is inactive against Ps. aeruginosa, Serratia, Citrobacter, Acinetobacter and Mycobacterium spp., as well as anaerobes, chlamydiae and ureaplasmas.
It is well absorbed and widely distributed when given orally. Absorption is not significantly affected by food, but ranitidine, sucralfate and some antacids or mineral supplements may interfere with absorption. After repeated doses of 400 mg every 12 h for 14 days, mean peak plasma levels reach 3.5–4.5 mg/L, a steady state being achieved in 3–4 days.
Clinical Use
1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-1,8- naphthyridine-3-carboxylic acid (Penetrex) is a quinolone with broad-spectrum antibacterial activity that is used primarily for the treatment of urinary tract infections and sexually transmitted diseases. Enoxacin has been approved for the treatment of uncomplicated gonococcal urethritis and has also been shown to be effective in chancroid caused by Haemophilus ducreyi. A single 400-mg dose is used for these indications. Enoxacin is also approved for the treatment of acute (uncomplicated) and chronic (complicated) urinary tract infections. Enoxacin is well absorbed following oral administration. Oral bioavailability approaches 98%. Concentrations of the drug in the kidneys, prostate, cervix, fallopian tubes, and myometrium typically exceed those in the plasma. More than 50% of the unchanged drug is excreted in the urine. Metabolism, largely catalyzed by cytochrome P450 enzymes in the liver, accounts for 15% to 20% of the orally administered dose of enoxacin. The relatively short elimination half-life of enoxacin dictates twice-a-day dosing for the treatment of urinary tract infections. Some cytochrome P450 isozymes, such as CYP 1A2, are inhibited by enoxacin, resulting in potentially important interactions with other drugs. For example, enoxacin has been reported to decrease theophylline clearance, causing increased plasma levels and increased toxicity. Enoxacin forms insoluble chelates with divalent metal ions present in antacids and hematinics, which reduce its oral bioavailability.
Enoxacin Preparation Products And Raw materials
Raw materials
1of4
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Indogulf Group | 91-22-23455220 | Maharashtra, India | 249 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3887 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| Maas Pharma Chemicals | +91-9958666224 | Delhi, India | 1607 | 58 | Inquiry |
| Samex Overseas | 08068441146Ext 740 | Gujarat, India | 124 | 58 | Inquiry |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | China | 2472 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21634 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29879 | 58 | Inquiry |
Related articles
- Toxicity of Enoxacin
- Enoxacin is an early-generation fluoroquinolone with the chemical formula (l-ethyl-6-fluoro-I,4-dihydro-4-oxo-7 piperazinyl)-I....
- Mar 24,2022





